
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10. Chapter 3: Electrochemistry Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Question 3.4:Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10. is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 3 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 3: Electrochemistry is very essencial for getting good marks in CBSE Board examinations
Question 3.4:Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer:For hydrogen electrode, ,
given that pH = 10
use formula [H+] = 10–pH
so that [H+] = 10−10 M
Electrode reaction will
H+ + e– →1/2 H2
Use the formula
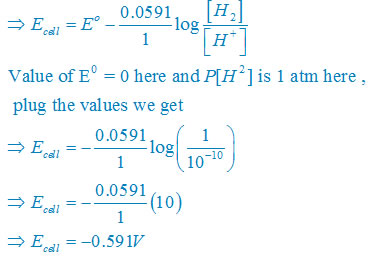
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.